The following workflow has been designed as teaching instructions for an introductory course to RNA-seq data analysis with DESeq2.
The course is designed for PhD students and will be given at the University of Münster from 10th to 21st of October 2016.
For questions or other comments, please contact me.
Go to exprAnalysis or this post for installation instructions.
For all functions, use the help pages to find out more about parameters and usage.
See Vignette for additional information.
library(exprAnalysis)
Input data
“As input, the DESeq2 package expects count data as obtained, e. g., from RNAseq or another high-throughput sequencing experiment, in the form of a matrix of integer values. The value in the i-th row and the j-th column of the matrix tells how many reads can be assigned to gene i in sample j.” Love et al., DESeq2 vignette
data("countmatrix")
Count data analysis with DESeq2
See DESeq2 Vignette for details.
- read in saved count matrix
- define experimental design
- convert to DESeq data set
Count matrix input
design <- gsub("(.*)(_[0-9])", "\\1", colnames(countmatrix))
ExpDesign <- data.frame(row.names=colnames(countmatrix), treatment = design)
data <- DESeq2::DESeqDataSetFromMatrix(countData = countmatrix, colData = ExpDesign, design = ~treatment)
## converting counts to integer mode
DESeq2
- optional, but recommended: remove genes with zero counts over all samples
- run DESeq
- Extracting transformed values
“While it is not necessary to pre-filter low count genes before running the DESeq2 functions, there are two reasons which make pre-filtering useful: by removing rows in which there are no reads or nearly no reads, we reduce the memory size of the dds data object and we increase the speed of the transformation and testing functions within DESeq2.” Love et al., DESeq2 vignette
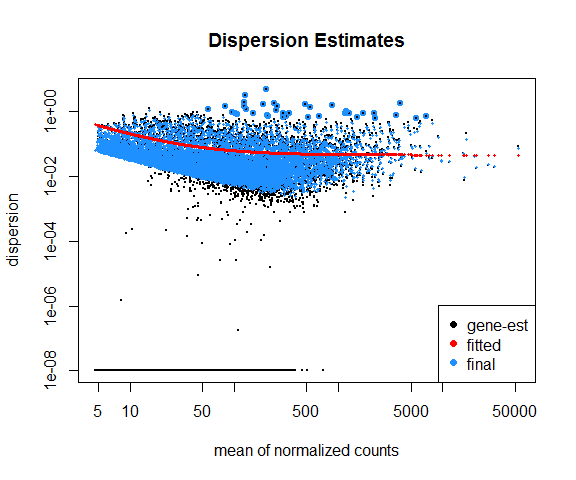
Note: the rlog transformation is provided for applications other than differential testing. For differential testing we recommend the DESeq function applied to raw counts, as described later in this workflow, which also takes into account the dependence of the variance of counts on the mean value during the dispersion estimation step.
For a quick first glance at the data, we can use pcaExplorer.
data <- data[rowSums(DESeq2::counts(data)) > 1, ]
data_DESeq <- DESeq2::DESeq(data)
## estimating size factors
## estimating dispersions
## gene-wise dispersion estimates
## mean-dispersion relationship
## final dispersion estimates
## fitting model and testing
expmatrix_DESeq <- DESeq2::rlog(data_DESeq, fitType="local")
expmatrix <- SummarizedExperiment::assay(expmatrix_DESeq)
library("pcaExplorer")
pcaExplorer(data_DESeq, expmatrix_DESeq)
Dispersion plot
DESeq2::plotDispEsts(data_DESeq, main="Dispersion Estimates")
Exploratory analysis of all genes
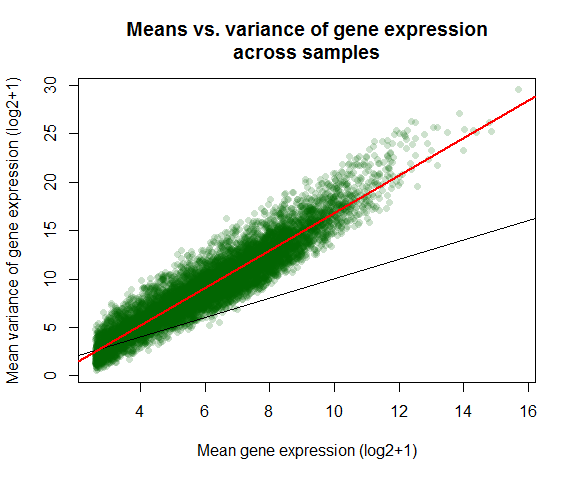
Variance vs mean gene expression across samples
Plots variance against mean gene expression across samples and calculates the correlation of a linear regression model.
var_vs_mean() uses the R package matrixStats.
var_vs_mean(countmatrix)
##
## Pearson's product-moment correlation
##
## data: log2(dispersion[, 1] + 1) and log2(dispersion[, 2] + 1)
## t = 281.2, df = 9998, p-value < 2.2e-16
## alternative hypothesis: true correlation is not equal to 0
## 95 percent confidence interval:
## 0.9399669 0.9443684
## sample estimates:
## cor
## 0.9422083
var_vs_mean(expmatrix)
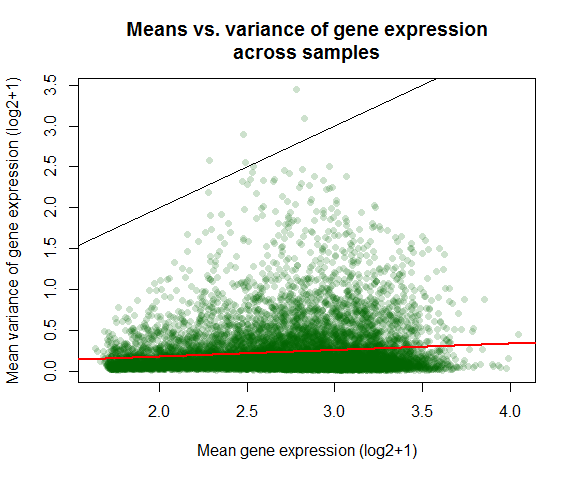
##
## Pearson's product-moment correlation
##
## data: log2(dispersion[, 1] + 1) and log2(dispersion[, 2] + 1)
## t = 12.133, df = 9998, p-value < 2.2e-16
## alternative hypothesis: true correlation is not equal to 0
## 95 percent confidence interval:
## 0.1010951 0.1397267
## sample estimates:
## cor
## 0.1204565
Intersample variances
library(corrgram)
Ctrl_cor <- expmatrix[,grep("Ctrl", colnames(expmatrix))]
corrgram::corrgram(Ctrl_cor, order=TRUE, lower.panel=corrgram::panel.pie,
upper.panel=corrgram::panel.pts, text.panel=corrgram::panel.txt,
main="Correlogram of controls")
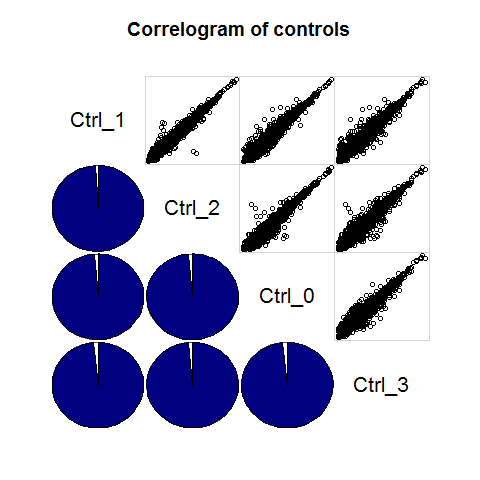
Repeat for other treatment groups
Principle Component Analysis
Uses functions from the R package pcaGoPromoter.
You can only plot the principle components using:
groups <- as.factor(c(rep("Ctrl",4), rep("TolLPS",4), rep("TolS100A8",4), rep("ActLPS",4)))
pca_plot(expmatrix, groups)
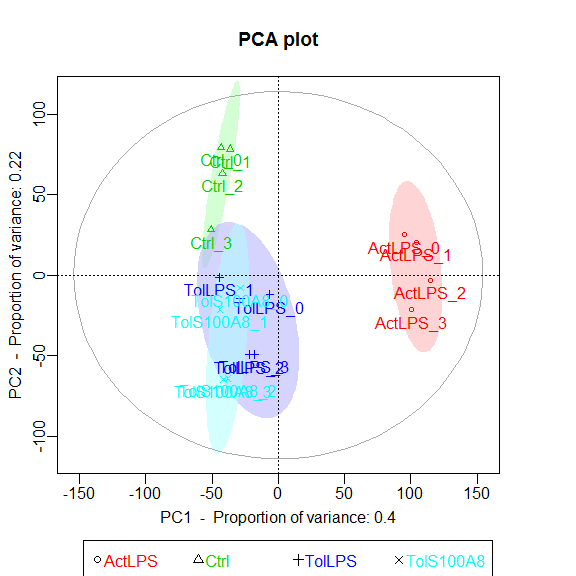
Or you can plot the principle components and calculate TF and GO term enrichments of genes (defaults to top 2.5%) with highest and lowest loadings. With this function, the ouput files are directly saved to .pdf and .txt (by default to working directory).
pca_plot_enrich(expmatrix, groups)
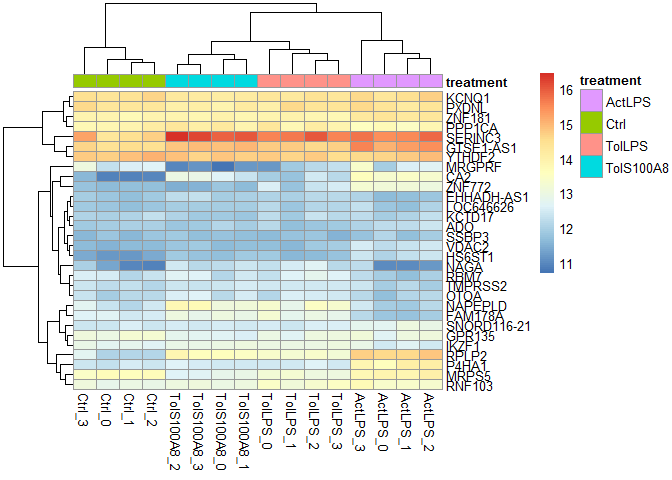
Heatmaps
heatmaps() uses the R package gplots.
Here, of the 30 most highly expressed genes.
select <- order(rowMeans(expmatrix),decreasing=TRUE)[1:30]
heatmaps(expmatrix[select,], samplecols = rep(c("#E41A1C", "#377EB8", "#4DAF4A", "#984EA3"), each=4))
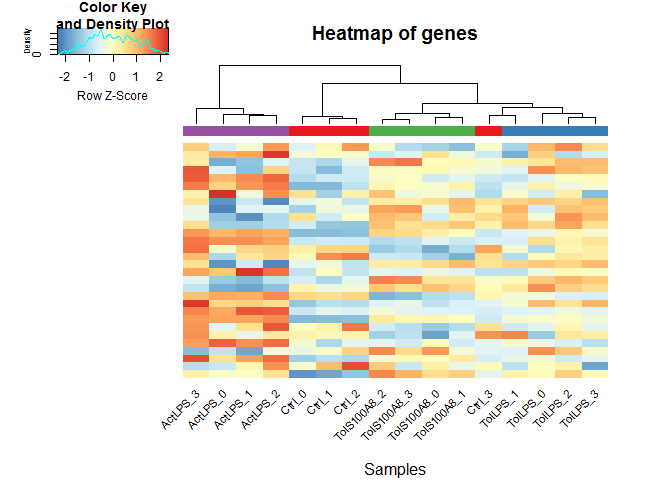
Heatmap function from DESeq2, using pheatmap:
library(pheatmap)
sampleDists <- dist(t(expmatrix))
sampleDistMatrix <- as.matrix(sampleDists)
rownames(sampleDistMatrix) <- paste(expmatrix_DESeq$treatment)
colnames(sampleDistMatrix) <- NULL
colors <- grDevices::colorRampPalette( rev(RColorBrewer::brewer.pal(9, "Blues")) )(255)
pheatmap::pheatmap(sampleDistMatrix,
clustering_distance_rows=sampleDists,
clustering_distance_cols=sampleDists,
col=colors)
df <- data.frame(treatment = SummarizedExperiment::colData(data_DESeq)[,c("treatment")], row.names = rownames(SummarizedExperiment::colData(data_DESeq)))
pheatmap::pheatmap(expmatrix[select,], cluster_rows=TRUE, show_rownames=TRUE, cluster_cols=TRUE, annotation_col=df)
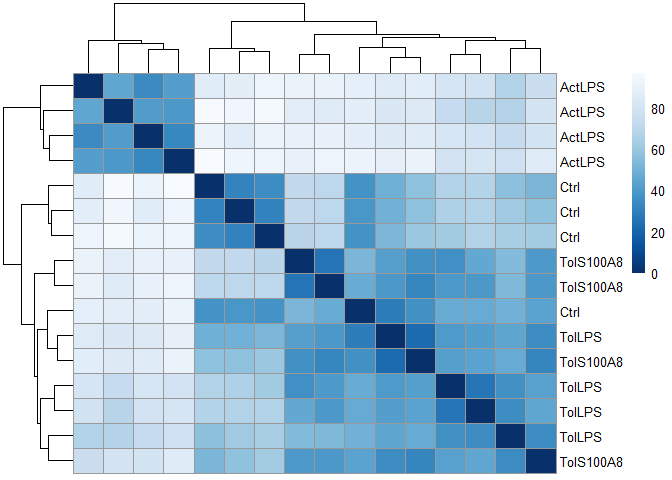
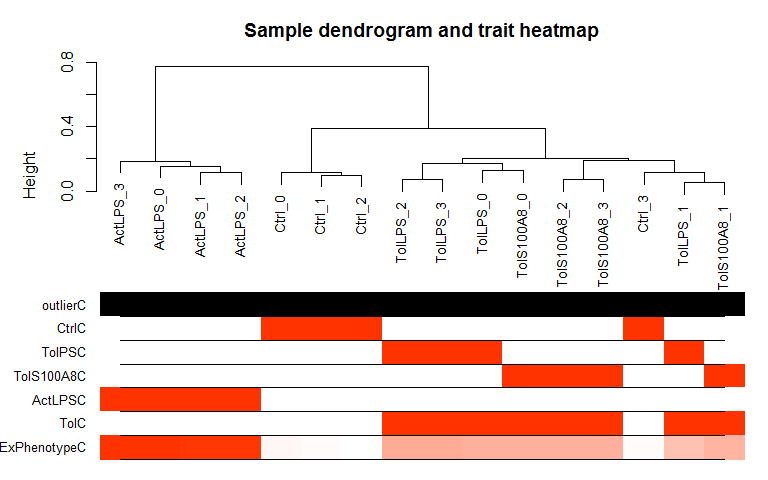
Hierarchical Clustering and outlier detection
Uses adjacency matrix function from the R package WGCNA and hierarchical clustering from the R package flashClust.
datTraits <- data.frame(Ctrl = c(rep(1, 4), rep(0,12)), TolPS = c(rep(0, 4), rep(1, 4),rep(0, 8)),
TolS100A8 = c(rep(0, 8), rep(1, 4), rep(0, 4)), ActLPS = c(rep(0, 12),rep(1, 4)),
Tol = c(rep(0, 4), rep(1, 8), rep(0, 4)),
ExPhenotype = c(stats::rnorm(4, 10, 1),stats::rnorm(8, 25, 1),stats::rnorm(4, 50, 1)),
row.names = colnames(expmatrix))
datExpr <- wgcna_sample_dendrogram(expmatrix, datTraits)
##
## Flagging genes and samples with too many missing values...
## ..step 1
## All genes are okay!
## All samples are okay!
# Optional: Remove outlier samples and repeats: All genes flagged for removal are saved to the object "remove_genes"
#head(remove_genes)
Differential expression analysis using DESeq2
For raw read count data.
contrast DE groups:
- lfc = treatment > Ctrl, - lfc = treatment < Ctrl p-value & p.adjust values of NA indicate outliers detected by Cook’s distance NA only for p.adjust means the gene is filtered by automatic independent filtering for having a low mean normalized count
Information about which variables and tests were used can be found by calling the function mcols, on the results object.
library(DESeq2)
library(ggplot2)
library(ggrepel)
# find possible contrasts with
DESeq2::resultsNames(data_DESeq)
## [1] "Intercept" "treatmentActLPS" "treatmentCtrl"
## [4] "treatmentTolLPS" "treatmentTolS100A8"
res <- DESeq2::results(data_DESeq, contrast=list("treatmentActLPS", "treatmentCtrl"), cooksCutoff = 0.99, independentFiltering = TRUE, alpha = 0.05, pAdjustMethod = "BH")
summary(res)
##
## out of 10000 with nonzero total read count
## adjusted p-value < 0.05
## LFC > 0 (up) : 2608, 26%
## LFC < 0 (down) : 2407, 24%
## outliers [1] : 175, 1.8%
## low counts [2] : 0, 0%
## (mean count < 5)
## [1] see 'cooksCutoff' argument of ?results
## [2] see 'independentFiltering' argument of ?results
mcols(res)$description
## [1] "mean of normalized counts for all samples"
## [2] "log2 fold change (MAP): treatmentActLPS vs treatmentCtrl"
## [3] "standard error: treatmentActLPS vs treatmentCtrl"
## [4] "Wald statistic: treatmentActLPS vs treatmentCtrl"
## [5] "Wald test p-value: treatmentActLPS vs treatmentCtrl"
## [6] "BH adjusted p-values"
# order results table by the smallest adjusted p value:
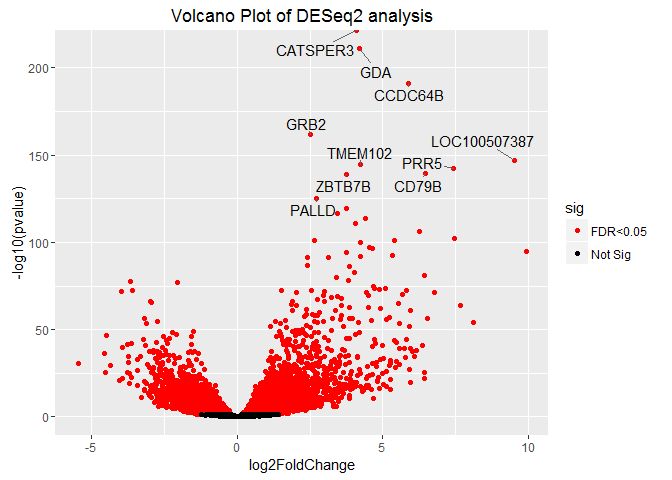
res <- res[order(res$padj),]
results = as.data.frame(dplyr::mutate(as.data.frame(res), sig=ifelse(res$padj<0.05, "FDR<0.05", "Not Sig")), row.names=rownames(res))
head(results)
## baseMean log2FoldChange lfcSE stat pvalue
## CATSPER3 2469.0350 4.094351 0.08958149 45.70532 0.000000e+00
## GDA 5578.3392 4.200987 0.13525887 31.05886 8.660849e-212
## CCDC64B 544.6121 5.878983 0.19906885 29.53241 1.104831e-191
## GRB2 1751.2594 2.506512 0.09224234 27.17312 1.350120e-162
## LOC100507387 716.7431 9.537221 0.36844495 25.88506 9.810056e-148
## TMEM102 1346.0139 4.244962 0.16534946 25.67267 2.360945e-145
## padj sig
## CATSPER3 0.000000e+00 FDR<0.05
## GDA 4.254642e-208 FDR<0.05
## CCDC64B 3.618323e-188 FDR<0.05
## GRB2 3.316233e-159 FDR<0.05
## LOC100507387 1.927676e-144 FDR<0.05
## TMEM102 3.866048e-142 FDR<0.05
DEgenes_DESeq <- results[which(abs(results$log2FoldChange) > log2(1.5) & results$padj < 0.05),]
p = ggplot2::ggplot(results, ggplot2::aes(log2FoldChange, -log10(pvalue))) +
ggplot2::geom_point(ggplot2::aes(col = sig)) +
ggplot2::scale_color_manual(values = c("red", "black")) +
ggplot2::ggtitle("Volcano Plot of DESeq2 analysis")
p + ggrepel::geom_text_repel(data=results[1:10, ], ggplot2::aes(label=rownames(results[1:10, ])))
## Warning: Removed 175 rows containing missing values (geom_point).
# If there aren't too many DE genes:
#p + geom_text_repel(data = dplyr::filter(results, padj<0.05), aes(label = rownames(results[1:10, ])))
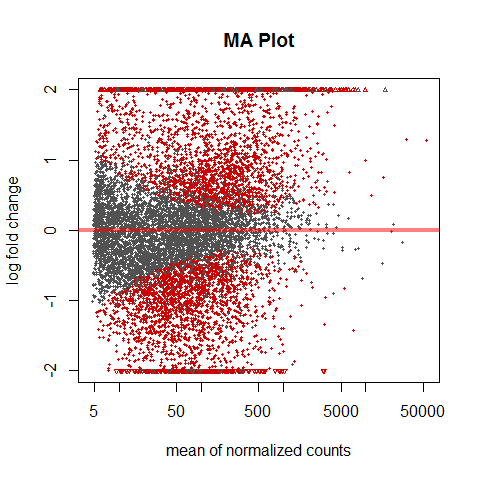
MA-plot
“These plots show the log2 fold changes from the treatment over the mean of normalized counts, i.e. the average of counts normalized by size factors. The left plot shows the “unshrunken” log2 fold changes, while the right plot, produced by the code above, shows the shrinkage of log2 fold changes resulting from the incorporation of zero-centered normal prior. The shrinkage is greater for the log2 fold change estimates from genes with low counts and high dispersion, as can be seen by the narrowing of spread of leftmost points in the right plot.” Love et al., DESeq2 vignette
DESeq2::plotMA(res, main="MA Plot", ylim=c(-2,2))
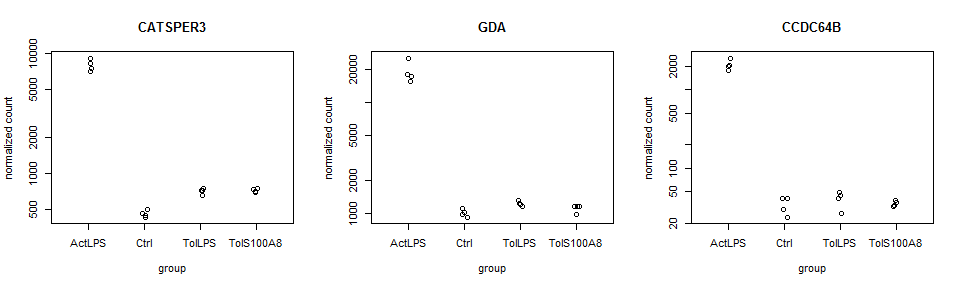
plotCounts
“It can also be useful to examine the counts of reads for a single gene across the groups. A simple function for making this plot is plotCounts, which normalizes counts by sequencing depth and adds a pseudocount of 1/2 to allow for log scale plotting. The counts are grouped by the variables in intgroup, where more than one variable can be specified.” Love et al., DESeq2 vignette
par(mfrow=c(1,3))
for (i in 1:3){
gene <- rownames(res)[i]
main = gene
DESeq2::plotCounts(data_DESeq, gene=gene, intgroup="treatment", main = main)
}
Gene annotations
Can be used to add e.g. ENTREZ ID, ENSEMBL ID, etc. to gene name.
results_anno <- geneAnnotations(input=results, keys=row.names(results), column=c("ENTREZID", "ENSEMBL"), keytype="SYMBOL", organism = "human")
##
## 'select()' returned 1:many mapping between keys and columns
## 'select()' returned 1:many mapping between keys and columns
head(results_anno)
## baseMean log2FoldChange lfcSE stat pvalue
## CATSPER3 2469.0350 4.094351 0.08958149 45.70532 0.000000e+00
## GDA 5578.3392 4.200987 0.13525887 31.05886 8.660849e-212
## CCDC64B 544.6121 5.878983 0.19906885 29.53241 1.104831e-191
## GRB2 1751.2594 2.506512 0.09224234 27.17312 1.350120e-162
## LOC100507387 716.7431 9.537221 0.36844495 25.88506 9.810056e-148
## TMEM102 1346.0139 4.244962 0.16534946 25.67267 2.360945e-145
## padj sig ENTREZID ENSEMBL
## CATSPER3 0.000000e+00 FDR<0.05 347732 ENSG00000152705
## GDA 4.254642e-208 FDR<0.05 9615 ENSG00000119125
## CCDC64B 3.618323e-188 FDR<0.05 146439 ENSG00000162069
## GRB2 3.316233e-159 FDR<0.05 2885 ENSG00000177885
## LOC100507387 1.927676e-144 FDR<0.05 100507387 ENSG00000182230
## TMEM102 3.866048e-142 FDR<0.05 284114 ENSG00000181284
Enrichment Analysis using clusterPofiler
See clusterProfiler instructions for details.
library(clusterProfiler)
## Loading required package: DOSE
library(org.Hs.eg.db)
## Loading required package: AnnotationDbi
OrgDb <- org.Hs.eg.db # can also be other organisms
geneList <- as.vector(results_anno$log2FoldChange)
names(geneList) <- results_anno$ENTREZID
gene <- na.omit(results_anno$ENTREZID)
# Group GO
ggo <- clusterProfiler::groupGO(gene = gene,
OrgDb = OrgDb,
ont = "BP",
level = 3,
readable = TRUE)
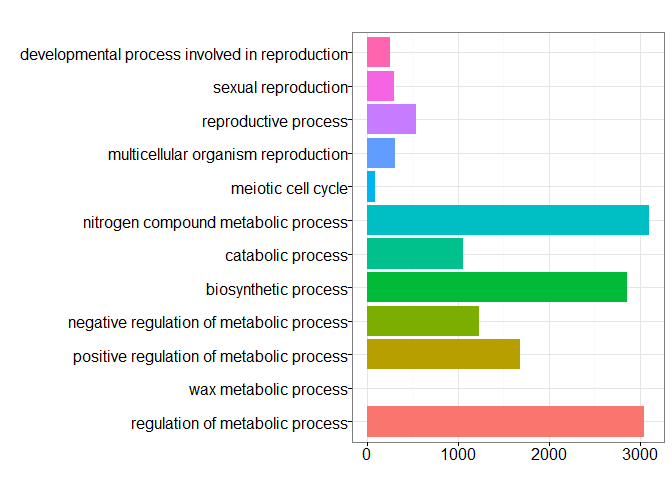
head(summary(ggo)[,-5])
## ID Description Count
## GO:0003006 GO:0003006 developmental process involved in reproduction 261
## GO:0019953 GO:0019953 sexual reproduction 303
## GO:0019954 GO:0019954 asexual reproduction 0
## GO:0022414 GO:0022414 reproductive process 547
## GO:0032504 GO:0032504 multicellular organism reproduction 317
## GO:0032505 GO:0032505 reproduction of a single-celled organism 0
## GeneRatio
## GO:0003006 261/9735
## GO:0019953 303/9735
## GO:0019954 0/9735
## GO:0022414 547/9735
## GO:0032504 317/9735
## GO:0032505 0/9735
barplot(ggo, drop=TRUE, showCategory=12)
# GO over-representation test
ego <- clusterProfiler::enrichGO(gene = gene,
OrgDb = OrgDb,
ont = "BP",
pAdjustMethod = "BH",
pvalueCutoff = 0.05,
qvalueCutoff = 0.05,
readable = TRUE)
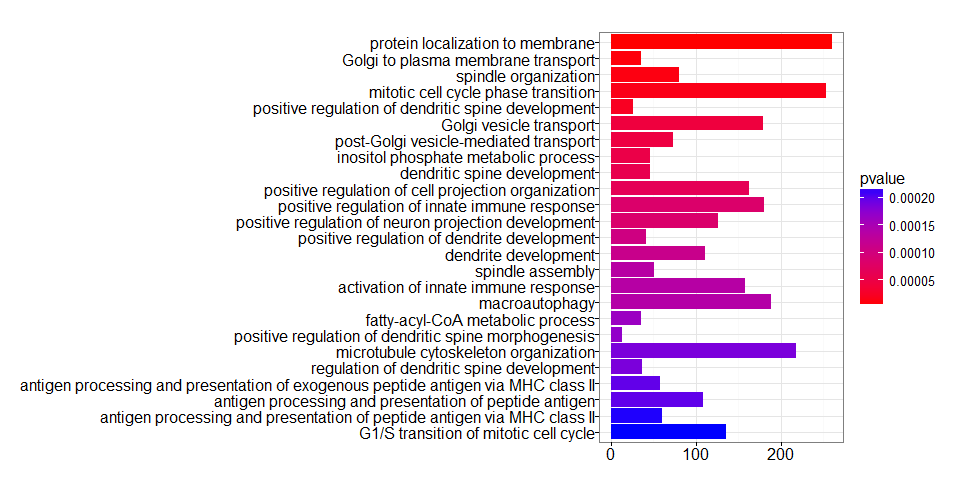
head(summary(ego)[,-8])
## ID Description
## GO:0072657 GO:0072657 protein localization to membrane
## GO:0006893 GO:0006893 Golgi to plasma membrane transport
## GO:0007051 GO:0007051 spindle organization
## GO:0044772 GO:0044772 mitotic cell cycle phase transition
## GO:0060999 GO:0060999 positive regulation of dendritic spine development
## GO:0048193 GO:0048193 Golgi vesicle transport
## GeneRatio BgRatio pvalue p.adjust qvalue Count
## GO:0072657 260/7259 487/16655 6.475400e-06 0.02201028 0.02035249 260
## GO:0006893 36/7259 48/16655 9.648441e-06 0.02201028 0.02035249 36
## GO:0007051 81/7259 130/16655 1.246381e-05 0.02201028 0.02035249 81
## GO:0044772 253/7259 477/16655 1.597263e-05 0.02201028 0.02035249 253
## GO:0060999 27/7259 34/16655 2.158731e-05 0.02379785 0.02200542 27
## GO:0048193 179/7259 329/16655 4.371899e-05 0.03540965 0.03274263 179
barplot(ego, showCategory=25)
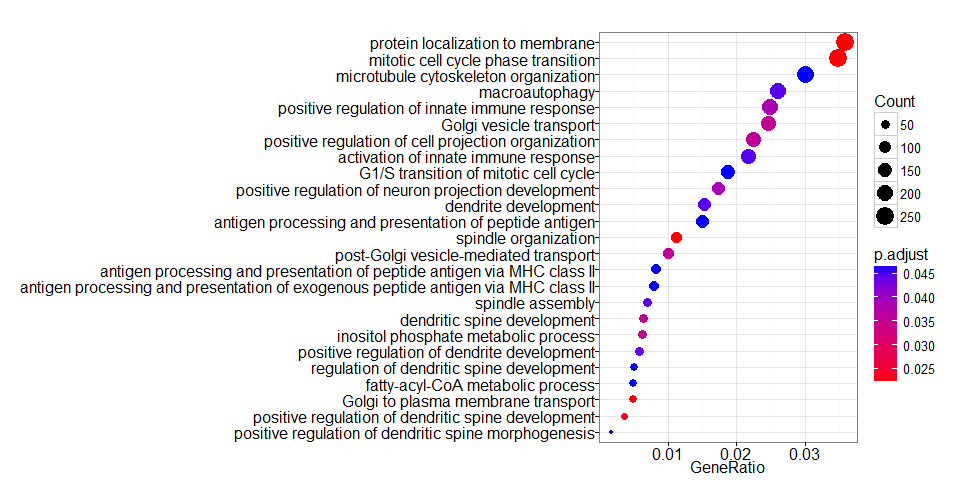
clusterProfiler::dotplot(ego, showCategory=25)
#clusterProfiler::plotGOgraph(ego)
## KEGG over-representation test
kk <- clusterProfiler::enrichKEGG(gene = gene,
organism = 'hsa',
pAdjustMethod = "BH",
pvalueCutoff = 0.05,
qvalueCutoff = 0.05)
head(summary(kk)[,-8])
## ID Description GeneRatio BgRatio
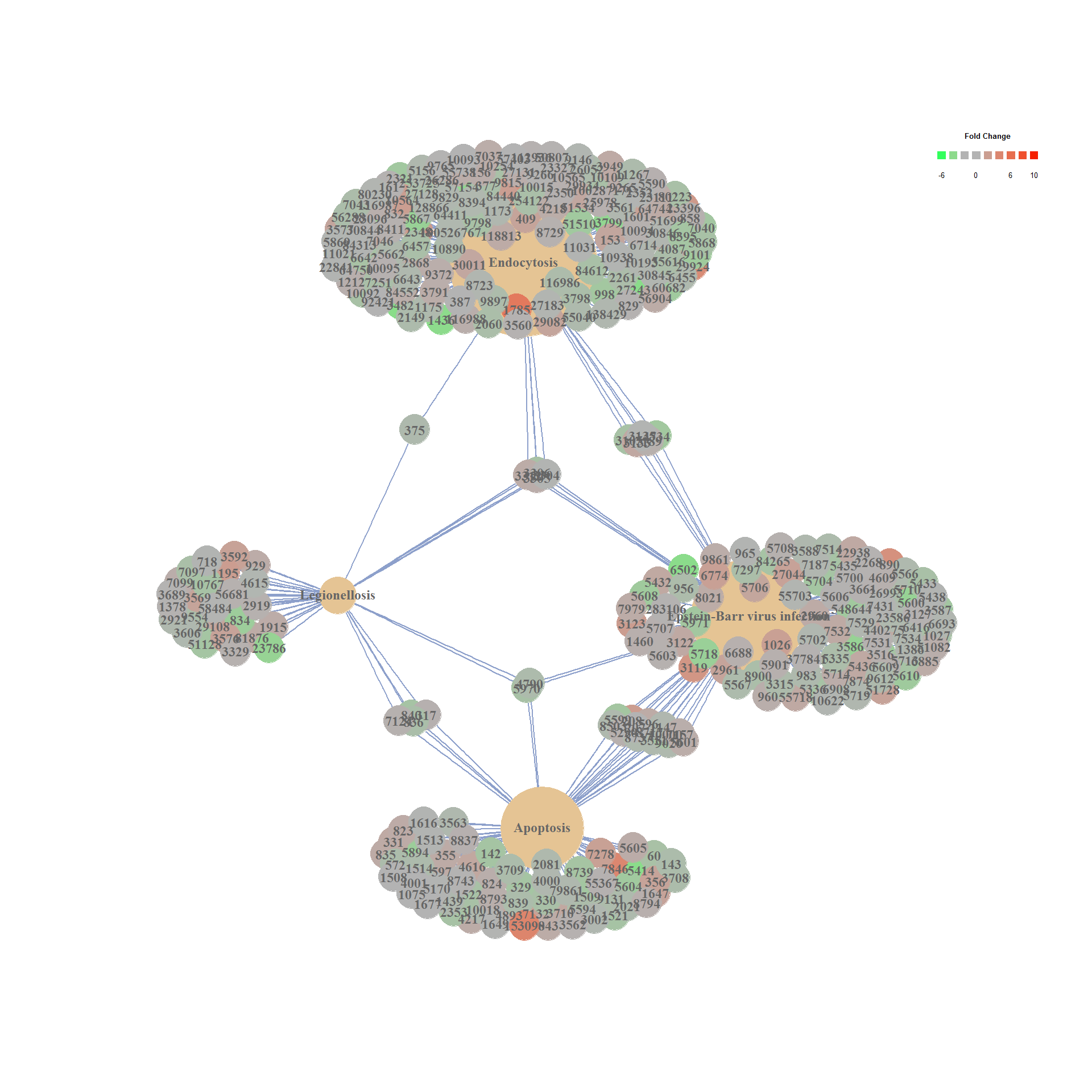
## hsa04144 hsa04144 Endocytosis 142/3071 260/7119
## hsa04210 hsa04210 Apoptosis 80/3071 140/7119
## hsa05169 hsa05169 Epstein-Barr virus infection 111/3071 204/7119
## hsa05134 hsa05134 Legionellosis 36/3071 55/7119
## pvalue p.adjust qvalue Count
## hsa04144 9.858484e-05 0.02858960 0.02345281 142
## hsa04210 5.292257e-04 0.04885909 0.04008042 80
## hsa05169 6.639562e-04 0.04885909 0.04008042 111
## hsa05134 6.739185e-04 0.04885909 0.04008042 36
cnetplot(kk, categorySize="geneNum", foldChange=geneList)