I’m an avid R user and rarely use anything else for data analysis and visualisations. But while R is my go-to, in some cases, Python might actually be a better alternative.
That’s why I wanted to see how R and Python fare in a one-on-one comparison of an analysis that’s representative of what I would typically work with.
Conclusions
All in all, the Python code could easily be translated into R and was comparable in length and simplicity between the two languages. While Python’s syntax is inherently cleaner/ tidier, we can use packages that implement piping in R and achieve similar results (even though Python’s dot-separated syntax is still much easier to type than using the piping operator of magrittr). For plotting and visualisation I still think that R’s ggplot2 is top of the line in both syntax, customizability and outcome (admittedly, I don’t know matplotlib as well as ggplot)! In terms of functionality, I couldn’t find major differences between the two languages and I would say they both have their merits. For me, R comes more natural as that is what I’m more fluent in, but I can see why Python holds an appeal too and I think I’ll make more of an effort to use both languages in my future projects.
R and Python
R and Python are both open-source languages used in a wide range of data analysis fields. Their main difference is that R has traditionally been geared towards statistical analysis, while Python is more generalist. Both comprise a large collection of packages for specific tasks and have a growing community that offers support and tutorials online.
For a nice overview of the languages respective strengths and weaknesses, check out Datacamp’s nice infographic.
Comparative analysis of genome data
To directly compare R and Python, I am following Zhuyi Xue’s “A Comprehensive Introduction To Your Genome With the SciPy Stack” (with some minor tweaks here and there). He gives a nice introduction to the data, so I will not repeat it here but focus on the comparison between the code lines.
For R, I am working with RStudio, for Python with Anaconda and Spyder.
Python:
For this analysis, we need the SciPy stack with pandas for data wrangling and matplotlib for visualisation. Anaconda already comes with all these packages that we need. Zhuyi Xue first imports pandas as “pd”, so that we can call pandas function by prefixing them with “pd.”.
import pandas as pd
R:
While the code could be replicated with base R, I prefer dplyr for data wrangling and ggplot2 for visualisation.
library(dplyr)
library(ggplot2)
Reading in data
Reading in data is straight forward in both R and Python. The code we need to read in the file is comparable between R and Python. Zhuyi Xue specified “compression = ‘gzip’”, but this would not have been necessary as the default is to infer it from the file suffix.
One big difference in the general syntax we can see here too: Boolean true/false values are written in all caps in R (TRUE/FALSE), while Python uses first letter capitalisation (True/False). The same prinicple applies to “none”.
Python:
df = pd.read_csv('Homo_sapiens.GRCh38.85.gff3.gz',
compression = 'gzip',
sep = '\t',
comment = '#',
low_memory = False,
header = None,
names = ['seqid', 'source', 'type', 'start', 'end', 'score', 'strand', 'phase', 'attributes'])
df.head()
R:
df <- read.csv("~/Documents/Github/Homo_sapiens.GRCh38.85.gff3.gz",
header = FALSE,
sep = "\t",
col.names = c('seqid', 'source', 'type', 'start', 'end', 'score', 'strand', 'phase', 'attributes'),
comment.char = "#")
head(df)
## seqid source type start end score strand phase
## 1 1 GRCh38 chromosome 1 248956422 . . .
## 2 1 . biological_region 10469 11240 1.3e+03 . .
## 3 1 . biological_region 10650 10657 0.999 + .
## 4 1 . biological_region 10655 10657 0.999 - .
## 5 1 . biological_region 10678 10687 0.999 + .
## 6 1 . biological_region 10681 10688 0.999 - .
## attributes
## 1 ID=chromosome:1;Alias=CM000663.2,chr1,NC_000001.11
## 2 external_name=oe %3D 0.79;logic_name=cpg
## 3 logic_name=eponine
## 4 logic_name=eponine
## 5 logic_name=eponine
## 6 logic_name=eponine
Examining data
- listing unique strings
The first thing we want to know from the data is how many unique entries there are in the “seqid” column.
Here, we can already see the main difference in syntax between R and Python:
Python concatenates the object name (“df) with the column name and the functions that we want to run on this column in a sequential manner, separated by a dot. Base R uses nested functions, where each function is called with “function_name()” and we specify columns with “object_name$column_name”.
However, both R and Python can also call columns in a dataframe with “[ ]” with the difference that Python per default subsets data columns df[“seqid”], while R always needs index specifications for rows and columns, separated by “,”: e.g. df[, “seqid”] would subset every row and only the column named “seqid”.
The sequential calling of functions is indeed very handy, it makes the code easier to read and understand than lots of interwoven functions and brackets. But while this isn’t the concept of base R, dplyr uses the magrittr principle of chaining functions with the pipe symbol “%>%”. Even though this symbol isn’t as easily typed, it’s functionality is often superior to base R, especially if you need to run many functions on a dataframe. However, with just or two functions, I usually keep to base R as it is shorter.
Python:
df.seqid.unique() # alternatively: df['seqid'].unique()
R:
unique(df$seqid)
## [1] 1 10 11 12 13 14
## [7] 15 16 17 18 19 2
## [13] 20 21 22 3 4 5
## [19] 6 7 8 9 GL000008.2 GL000009.2
## [25] GL000194.1 GL000195.1 GL000205.2 GL000208.1 GL000213.1 GL000214.1
## [31] GL000216.2 GL000218.1 GL000219.1 GL000220.1 GL000221.1 GL000224.1
## [37] GL000225.1 GL000226.1 KI270302.1 KI270303.1 KI270304.1 KI270305.1
## [43] KI270310.1 KI270311.1 KI270312.1 KI270315.1 KI270316.1 KI270317.1
## [49] KI270320.1 KI270322.1 KI270329.1 KI270330.1 KI270333.1 KI270334.1
## [55] KI270335.1 KI270336.1 KI270337.1 KI270338.1 KI270340.1 KI270362.1
## [61] KI270363.1 KI270364.1 KI270366.1 KI270371.1 KI270372.1 KI270373.1
## [67] KI270374.1 KI270375.1 KI270376.1 KI270378.1 KI270379.1 KI270381.1
## [73] KI270382.1 KI270383.1 KI270384.1 KI270385.1 KI270386.1 KI270387.1
## [79] KI270388.1 KI270389.1 KI270390.1 KI270391.1 KI270392.1 KI270393.1
## [85] KI270394.1 KI270395.1 KI270396.1 KI270411.1 KI270412.1 KI270414.1
## [91] KI270417.1 KI270418.1 KI270419.1 KI270420.1 KI270422.1 KI270423.1
## [97] KI270424.1 KI270425.1 KI270429.1 KI270435.1 KI270438.1 KI270442.1
## [103] KI270448.1 KI270465.1 KI270466.1 KI270467.1 KI270468.1 KI270507.1
## [109] KI270508.1 KI270509.1 KI270510.1 KI270511.1 KI270512.1 KI270515.1
## [115] KI270516.1 KI270517.1 KI270518.1 KI270519.1 KI270521.1 KI270522.1
## [121] KI270528.1 KI270529.1 KI270530.1 KI270538.1 KI270539.1 KI270544.1
## [127] KI270548.1 KI270579.1 KI270580.1 KI270581.1 KI270582.1 KI270583.1
## [133] KI270584.1 KI270587.1 KI270588.1 KI270589.1 KI270590.1 KI270591.1
## [139] KI270593.1 KI270706.1 KI270707.1 KI270708.1 KI270709.1 KI270710.1
## [145] KI270711.1 KI270712.1 KI270713.1 KI270714.1 KI270715.1 KI270716.1
## [151] KI270717.1 KI270718.1 KI270719.1 KI270720.1 KI270721.1 KI270722.1
## [157] KI270723.1 KI270724.1 KI270725.1 KI270726.1 KI270727.1 KI270728.1
## [163] KI270729.1 KI270730.1 KI270731.1 KI270732.1 KI270733.1 KI270734.1
## [169] KI270735.1 KI270736.1 KI270737.1 KI270738.1 KI270739.1 KI270740.1
## [175] KI270741.1 KI270742.1 KI270743.1 KI270744.1 KI270745.1 KI270746.1
## [181] KI270747.1 KI270748.1 KI270749.1 KI270750.1 KI270751.1 KI270752.1
## [187] KI270753.1 KI270754.1 KI270755.1 KI270756.1 KI270757.1 MT
## [193] X Y
## 194 Levels: 1 10 11 12 13 14 15 16 17 18 19 2 20 21 22 3 4 5 6 7 8 ... Y
# with dplyr:
# df %>% select(seqid) %>% unique
- how many unique seqids are there?
To get the number of unique entries in the “seqid” column, we simply need to append “.shape” to the above Python code. In R, we can either wrap the above R code with the “length()” function or use dplyr and piping instead. If we use the latter, we need to use the “nrow()” function because base R returns a vector, while dplyr returns a dataframe. Here, we can see that with two functions, using dplyr is still a bit more code but it already looks much tidier.
Python:
df.seqid.unique().shape
R:
length(unique(df$seqid))
## [1] 194
# with dplyr:
# df %>% select(seqid) %>% unique %>% nrow
- counting occurrences
To count the frequencies of each unique entry in the “source” column, we use the “value_counts()” function in Python and the “table()” function in R. These two functions differ in how they sort the output table: value_counts() sorts by decreasing frequency, while R alphabetically sorts the variables. To order the data as in Python, we need to add the “sort()” function to our R code.
Python:
df.source.value_counts()
R:
# table(df$source) is per default ordered alphabetically, if we want it ordered by decreasing counts, like with Python:
sort(table(df$source), decreasing = TRUE)
##
## havana ensembl_havana ensembl . mirbase
## 1441093 745065 228212 182510 4701
## GRCh38 insdc
## 194 74
# dplyr:
# df %>% select(source) %>% table %>% sort(decreasing = TRUE)
How Much of the Genome Is Incomplete?
- subsetting a dataframe
We are now subsetting our original dataframe and assign it a new object name with “ = “ or “ <- “.
To subset the dataframe to keep only rows which say “GRCh38” in the “source” column, ther are several ways to do this in R: the way that would be directly comparable to how it was done in Python would be to also use the square bracket indexing. However, there are two solutions which are more elegant: 1) base R’s “subset()” or dplyr’s “filter()” function. But with this short example, there is no big difference between the three.
Python’s “shape” gives us the same information as R’s “dim()” function: how many rows and columns our dataframe has.
To preview a random subset of 10 rows from our dataframe, we use Python’s “sample()” and dplyr’s “sample_n()” function.
Python:
gdf = df[df.source == 'GRCh38']
gdf.shape
gdf.sample(10)
R:
gdf <- df[df$source == "GRCh38", ]
# alternatively:
# gdf <- subset(df, source == "GRCh38")
# dplyr:
# gdf <- df %>% filter(source == "GRCh38")
# get number of rows and columns
dim(gdf)
## [1] 194 9
# randomly sample 10 rows for observation
sample_n(gdf, 10)
## seqid source type start end score strand phase
## 2511484 KI270375.1 GRCh38 supercontig 1 2378 . . .
## 2511545 KI270466.1 GRCh38 supercontig 1 1233 . . .
## 2511473 KI270337.1 GRCh38 supercontig 1 1121 . . .
## 2511504 KI270411.1 GRCh38 supercontig 1 2646 . . .
## 2511487 KI270379.1 GRCh38 supercontig 1 1045 . . .
## 483371 12 GRCh38 chromosome 1 133275309 . . .
## 2511494 KI270387.1 GRCh38 supercontig 1 1537 . . .
## 674768 14 GRCh38 chromosome 1 107043718 . . .
## 2511493 KI270386.1 GRCh38 supercontig 1 1788 . . .
## 2511505 KI270412.1 GRCh38 supercontig 1 1179 . . .
## attributes
## 2511484 ID=supercontig:KI270375.1;Alias=chrUn_KI270375v1,NT_187493.1
## 2511545 ID=supercontig:KI270466.1;Alias=chrUn_KI270466v1,NT_187421.1
## 2511473 ID=supercontig:KI270337.1;Alias=chrUn_KI270337v1,NT_187466.1
## 2511504 ID=supercontig:KI270411.1;Alias=chrUn_KI270411v1,NT_187409.1
## 2511487 ID=supercontig:KI270379.1;Alias=chrUn_KI270379v1,NT_187472.1
## 483371 ID=chromosome:12;Alias=CM000674.2,chr12,NC_000012.12
## 2511494 ID=supercontig:KI270387.1;Alias=chrUn_KI270387v1,NT_187475.1
## 674768 ID=chromosome:14;Alias=CM000676.2,chr14,NC_000014.9
## 2511493 ID=supercontig:KI270386.1;Alias=chrUn_KI270386v1,NT_187480.1
## 2511505 ID=supercontig:KI270412.1;Alias=chrUn_KI270412v1,NT_187408.1
- creating new columns and performing calculations
Now we want to create a new column called “length”. It should contain the gene lengths, i.e. the distance in base pairs between the start and end point on the chromosomes (“start” and “end” columns). In R we don’t need to copy the dataframe first but the rest of the code is very similar: we define a new column name for the dataframe and assign its value by subtracting the values of the “start” from the values of the “end” column (+1). Here, we can see again that in Python we use a dot to define columns, while R uses the Dollar sign.
The sum of all lengths is calculated with the “sum()” function in both languages.
Python:
gdf = gdf.copy()
gdf['length'] = gdf.end - gdf.start + 1
gdf.length.sum()
R:
gdf$length <- gdf$end - gdf$start + 1
sum(gdf$length)
## [1] 3096629726
Next, we want to calculating the proportion of the genome that is not on main chromosome assemblies. For that, we first define a character string with the main chromosomes: 1 to 23, X, Y and MT (mitochondrial chromosome). Defining this string is a bit easier in R.
We will use this string to calculate the sum of lengths of the subsetted dataframe and divide it by the sum of lengths of the whole dataframe. For subsetting, we use the “isin()” function of Python, which corresponds to R’s “%in%”.
Python:
chrs = [str(_) for _ in range(1, 23)] + ['X', 'Y', 'MT']
gdf[-gdf.seqid.isin(chrs)].length.sum() / gdf.length.sum()
# or
gdf[(gdf['type'] == 'supercontig')].length.sum() / gdf.length.sum()
R:
chrs <- c(1:23, "X", "Y", "MT")
sum(subset(gdf, !seqid %in% chrs)$length) / sum(gdf$length)
## [1] 0.003702192
How Many Genes Are There?
Here, we are again using the same functions as above for subsetting the dataframe, asking for its dimensions, printing 10 random lines and asking for the frequencies of each unique item in the “type” column. For the latter I am using base R over dplyr, because it’s faster to type.
Python:
edf = df[df.source.isin(['ensembl', 'havana', 'ensembl_havana'])]
edf.shape
edf.sample(10)
edf.type.value_counts()
R:
edf <- subset(df, source %in% c("ensembl", "havana", "ensembl_havana"))
dim(edf)
## [1] 2414370 9
sample_n(edf, 10)
## seqid source type start end score
## 2548897 X ensembl three_prime_UTR 67723842 67730618 .
## 790670 15 ensembl_havana CDS 42352030 42352139 .
## 1298369 19 havana CDS 38942683 38942751 .
## 189728 1 ensembl_havana CDS 186304108 186304257 .
## 1080599 17 havana exon 44401337 44401453 .
## 1562968 20 ensembl_havana exon 2964272 2964350 .
## 1965100 5 ensembl transcript 144087 144197 .
## 2581554 X havana exon 147927670 147928807 .
## 785399 15 havana exon 40611478 40611511 .
## 1417830 2 ensembl_havana CDS 71520178 71520208 .
## strand phase
## 2548897 + .
## 790670 + 2
## 1298369 - 0
## 189728 + 2
## 1080599 - .
## 1562968 + .
## 1965100 - .
## 2581554 + .
## 785399 + .
## 1417830 + 0
## attributes
## 2548897 Parent=transcript:ENST00000612452
## 790670 ID=CDS:ENSP00000326227;Parent=transcript:ENST00000318010;protein_id=ENSP00000326227
## 1298369 ID=CDS:ENSP00000472465;Parent=transcript:ENST00000599996;protein_id=ENSP00000472465
## 189728 ID=CDS:ENSP00000356453;Parent=transcript:ENST00000367483;protein_id=ENSP00000356453
## 1080599 Parent=transcript:ENST00000585614;Name=ENSE00002917300;constitutive=0;ensembl_end_phase=2;ensembl_phase=2;exon_id=ENSE00002917300;rank=7;version=1
## 1562968 Parent=transcript:ENST00000216877;Name=ENSE00003603687;constitutive=1;ensembl_end_phase=1;ensembl_phase=-1;exon_id=ENSE00003603687;rank=4;version=1
## 1965100 ID=transcript:ENST00000362670;Parent=gene:ENSG00000199540;Name=Y_RNA.43-201;biotype=misc_RNA;tag=basic;transcript_id=ENST00000362670;transcript_support_level=NA;version=1
## 2581554 Parent=transcript:ENST00000620828;Name=ENSE00003724969;constitutive=0;ensembl_end_phase=-1;ensembl_phase=-1;exon_id=ENSE00003724969;rank=1;version=1
## 785399 Parent=transcript:ENST00000399668;Name=ENSE00003659959;constitutive=0;ensembl_end_phase=2;ensembl_phase=1;exon_id=ENSE00003659959;rank=7;version=1
## 1417830 ID=CDS:ENSP00000398305;Parent=transcript:ENST00000429174;protein_id=ENSP00000398305
sort(table(edf$type), decreasing = TRUE)
##
## exon CDS
## 1180596 704604
## five_prime_UTR three_prime_UTR
## 142387 133938
## transcript gene
## 96375 42470
## processed_transcript aberrant_processed_transcript
## 28228 26944
## NMD_transcript_variant lincRNA
## 13761 13247
## processed_pseudogene lincRNA_gene
## 10722 7533
## pseudogene RNA
## 3049 2221
## snRNA snRNA_gene
## 1909 1909
## snoRNA snoRNA_gene
## 956 944
## pseudogenic_transcript rRNA
## 737 549
## rRNA_gene miRNA
## 549 302
## V_gene_segment J_gene_segment
## 216 158
## VD_gene_segment C_gene_segment
## 37 29
## biological_region chromosome
## 0 0
## miRNA_gene mt_gene
## 0 0
## supercontig
## 0
Now we want to subset the dataframe to rows with the attribute “gene” in the “type” column, look at 10 random lines from the “attributes” column and get the dataframe dimensions.
Python:
ndf = edf[edf.type == 'gene']
ndf = ndf.copy()
ndf.sample(10).attributes.values
ndf.shape
R:
ndf <- subset(edf, type == "gene")
sample_n(ndf, 10)$attributes
## [1] ID=gene:ENSG00000170949;Name=ZNF160;biotype=protein_coding;description=zinc finger protein 160 [Source:HGNC Symbol%3BAcc:HGNC:12948];gene_id=ENSG00000170949;havana_gene=OTTHUMG00000182854;havana_version=3;logic_name=ensembl_havana_gene;version=17
## [2] ID=gene:ENSG00000215088;Name=RPS5P3;biotype=processed_pseudogene;description=ribosomal protein S5 pseudogene 3 [Source:HGNC Symbol%3BAcc:HGNC:10427];gene_id=ENSG00000215088;havana_gene=OTTHUMG00000065532;havana_version=1;logic_name=havana;version=3
## [3] ID=gene:ENSG00000249077;Name=RP11-478C1.8;biotype=processed_pseudogene;gene_id=ENSG00000249077;havana_gene=OTTHUMG00000160290;havana_version=1;logic_name=havana;version=1
## [4] ID=gene:ENSG00000110619;Name=CARS;biotype=protein_coding;description=cysteinyl-tRNA synthetase [Source:HGNC Symbol%3BAcc:HGNC:1493];gene_id=ENSG00000110619;havana_gene=OTTHUMG00000010927;havana_version=9;logic_name=ensembl_havana_gene;version=16
## [5] ID=gene:ENSG00000168010;Name=ATG16L2;biotype=protein_coding;description=autophagy related 16 like 2 [Source:HGNC Symbol%3BAcc:HGNC:25464];gene_id=ENSG00000168010;havana_gene=OTTHUMG00000167961;havana_version=2;logic_name=ensembl_havana_gene;version=10
## [6] ID=gene:ENSG00000055957;Name=ITIH1;biotype=protein_coding;description=inter-alpha-trypsin inhibitor heavy chain 1 [Source:HGNC Symbol%3BAcc:HGNC:6166];gene_id=ENSG00000055957;havana_gene=OTTHUMG00000150312;havana_version=9;logic_name=ensembl_havana_gene;version=10
## [7] ID=gene:ENSG00000258781;Name=RP11-496I2.4;biotype=unprocessed_pseudogene;gene_id=ENSG00000258781;havana_gene=OTTHUMG00000170502;havana_version=2;logic_name=havana;version=2
## [8] ID=gene:ENSG00000157965;Name=SSX8;biotype=unprocessed_pseudogene;description=SSX family member 8 [Source:HGNC Symbol%3BAcc:HGNC:19654];gene_id=ENSG00000157965;havana_gene=OTTHUMG00000021573;havana_version=4;logic_name=havana;version=11
## [9] ID=gene:ENSG00000273489;Name=RP11-180C16.1;biotype=antisense;gene_id=ENSG00000273489;havana_gene=OTTHUMG00000186336;havana_version=1;logic_name=havana;version=1
## [10] ID=gene:ENSG00000279532;Name=CTB-96E2.6;biotype=TEC;gene_id=ENSG00000279532;havana_gene=OTTHUMG00000179414;havana_version=1;logic_name=havana;version=1
## 1623077 Levels: external_name=Ala;logic_name=trnascan ...
dim(ndf)
## [1] 42470 9
- extracting gene information from attributes field
I don’t know if there is an easier way in Python but in R we don’t need to create big helper functions around it. We can simply use “gsub()” with defining the regular expression for what we want to extract. This makes the R code much shorter and easier to understand! We then drop the original “attributes” column.
To have a look at the dataframe, we use “head()” this time.
Python:
import re
RE_GENE_NAME = re.compile(r'Name=(?P<gene_name>.+?);')
def extract_gene_name(attributes_str):
res = RE_GENE_NAME.search(attributes_str)
return res.group('gene_name')
ndf['gene_name'] = ndf.attributes.apply(extract_gene_name)
RE_GENE_ID = re.compile(r'gene_id=(?P<gene_id>ENSG.+?);')
def extract_gene_id(attributes_str):
res = RE_GENE_ID.search(attributes_str)
return res.group('gene_id')
ndf['gene_id'] = ndf.attributes.apply(extract_gene_id)
RE_DESC = re.compile('description=(?P<desc>.+?);')
def extract_description(attributes_str):
res = RE_DESC.search(attributes_str)
if res is None:
return ''
else:
return res.group('desc')
ndf['desc'] = ndf.attributes.apply(extract_description)
ndf.drop('attributes', axis=1, inplace=True)
ndf.head()
R:
ndf$gene_name <- gsub("(.*Name=)(.*?)(;biotype.*)", "\\2", ndf$attributes)
ndf$gene_id <- gsub("(ID=gene:)(.*?)(;Name.*)", "\\2", ndf$attributes)
ndf$desc <- gsub("(.*description=)(.*?)(;.*)", "\\2", ndf$attributes)
# some genes don't have a description
ndf$desc <- ifelse(grepl("^ID=.*", ndf$desc), "", ndf$desc)
ndf <- subset(ndf, select = -attributes)
head(ndf)
## seqid source type start end score strand phase gene_name
## 17 1 havana gene 11869 14409 . + . DDX11L1
## 29 1 havana gene 14404 29570 . - . WASH7P
## 72 1 havana gene 52473 53312 . + . OR4G4P
## 75 1 havana gene 62948 63887 . + . OR4G11P
## 78 1 ensembl_havana gene 69091 70008 . + . OR4F5
## 109 1 havana gene 131025 134836 . + . CICP27
## gene_id
## 17 ENSG00000223972
## 29 ENSG00000227232
## 72 ENSG00000268020
## 75 ENSG00000240361
## 78 ENSG00000186092
## 109 ENSG00000233750
## desc
## 17 DEAD/H-box helicase 11 like 1 [Source:HGNC Symbol%3BAcc:HGNC:37102]
## 29 WAS protein family homolog 7 pseudogene [Source:HGNC Symbol%3BAcc:HGNC:38034]
## 72 olfactory receptor family 4 subfamily G member 4 pseudogene [Source:HGNC Symbol%3BAcc:HGNC:14822]
## 75 olfactory receptor family 4 subfamily G member 11 pseudogene [Source:HGNC Symbol%3BAcc:HGNC:31276]
## 78 olfactory receptor family 4 subfamily F member 5 [Source:HGNC Symbol%3BAcc:HGNC:14825]
## 109 capicua transcriptional repressor pseudogene 27 [Source:HGNC Symbol%3BAcc:HGNC:48835]
Next, we want to know how many unique gene names and gene IDs there are. As above we use the “unique()”, “shape()” (Python) and “length()” (R) functions.
The count table for gene names can again be obtained with R’s “table()” function, even though Zhuyi Xue uses a slightly different approach: he first groups the “gene_name” column, then counts and sorts.
Finally, we can calculate the proportion of genes that have more than appear more than once and we can have a closer look at the SCARNA20 gene.
Python:
ndf.shape
ndf.gene_id.unique().shape
ndf.gene_name.unique().shape
count_df = ndf.groupby('gene_name').count().ix[:, 0].sort_values().ix[::-1]
count_df.head(10)
count_df[count_df > 1].shape
count_df.shape
count_df[count_df > 1].shape[0] / count_df.shape[0]
ndf[ndf.gene_name == 'SCARNA20']
R:
dim(ndf)
## [1] 42470 11
length(unique(ndf$gene_id))
## [1] 42470
length(unique(ndf$gene_name))
## [1] 42387
count_df <- sort(table(ndf$gene_name), decreasing = TRUE)
head(count_df, n = 10)
##
## SCARNA20 SCARNA16 SCARNA17 SCARNA11
## 7 6 5 4
## SCARNA15 SCARNA21 Clostridiales-1 SCARNA4
## 4 4 3 3
## ACTR3BP2 AGBL1
## 2 2
length(count_df[count_df > 1])
## [1] 63
length(count_df)
## [1] 42387
length(count_df[count_df > 1]) / length(count_df)
## [1] 0.001486305
ndf[ndf$gene_name == "SCARNA20", ]
## seqid source type start end score strand phase
## 179400 1 ensembl gene 171768070 171768175 . + .
## 201038 1 ensembl gene 204727991 204728106 . + .
## 349204 11 ensembl gene 8555016 8555146 . + .
## 718521 14 ensembl gene 63479272 63479413 . + .
## 837234 15 ensembl gene 75121536 75121666 . - .
## 1039875 17 ensembl gene 28018770 28018907 . + .
## 1108216 17 ensembl gene 60231516 60231646 . - .
## gene_name gene_id
## 179400 SCARNA20 ENSG00000253060
## 201038 SCARNA20 ENSG00000251861
## 349204 SCARNA20 ENSG00000252778
## 718521 SCARNA20 ENSG00000252800
## 837234 SCARNA20 ENSG00000252722
## 1039875 SCARNA20 ENSG00000251818
## 1108216 SCARNA20 ENSG00000252577
## desc
## 179400 Small Cajal body specific RNA 20 [Source:RFAM%3BAcc:RF00601]
## 201038 Small Cajal body specific RNA 20 [Source:RFAM%3BAcc:RF00601]
## 349204 Small Cajal body specific RNA 20 [Source:RFAM%3BAcc:RF00601]
## 718521 Small Cajal body specific RNA 20 [Source:RFAM%3BAcc:RF00601]
## 837234 Small Cajal body specific RNA 20 [Source:RFAM%3BAcc:RF00601]
## 1039875 Small Cajal body specific RNA 20 [Source:RFAM%3BAcc:RF00601]
## 1108216 small Cajal body-specific RNA 20 [Source:HGNC Symbol%3BAcc:HGNC:32578]
# dplyr:
# ndf %>% filter(gene_name == "SCARNA20")
How Long Is a Typical Gene?
To calculate gene lengths we use the same code as before. R’s summary() is not exactly the same as Python’s describe() but it’s close enough.
Python:
ndf['length'] = ndf.end - ndf.start + 1
ndf.length.describe()
R:
ndf$length <- ndf$end - ndf$start + 1
summary(ndf$length)
## Min. 1st Qu. Median Mean 3rd Qu. Max.
## 8 884 5170 35830 30550 2305000
Now we produce the first plot, showing a histogram of gene length. In base R you can’t really plot a histogram with logarithmic y-axis scales (at least not without manually tweaking the hist() output but it isn’t recommended anyway because 0 will become -Inf). But we can do it easily with ggplot2 with “scale_y_log10()”. The code we need for ggplot2 is a bit longer than with matplotlib. We could of course further customize our plot but for now, let’s keep it simple.
Python:
import matplotlib as plt
ndf.length.plot(kind='hist', bins=50, logy=True)
plt.show()
R:
ndf %>% ggplot(aes(x = length)) +
geom_histogram(bins = 50, fill = "blue") +
scale_y_log10()
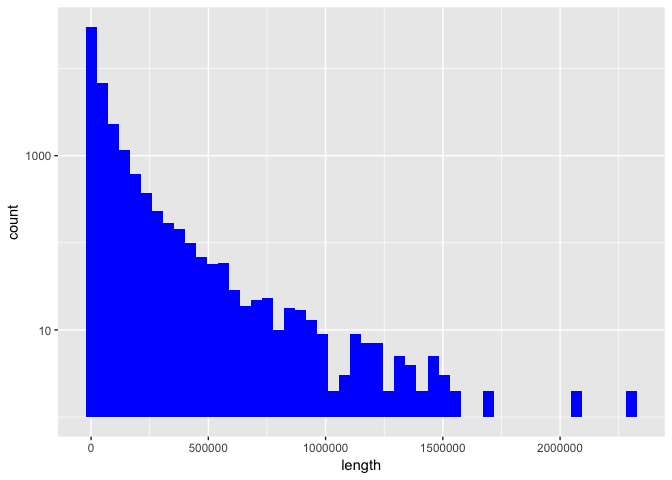
Now, we subset the dataframe to keep only rows where the “length” column contains values bigger than 2 million and order it by descending gene length. To see the shortest genes, we order the original dataframe and view the first 6 rows. Instead of “sort()”, we use dplyr’s “arrange()” function this time (I didn’t use it before because it can only be applied to dataframes).
Python:
ndf[ndf.length > 2e6].sort_values('length').ix[::-1]
ndf.sort_values('length').head()
R:
ndf %>% filter(length > 2e6) %>% arrange(desc(length))
## seqid source type start end score strand phase
## 1 7 ensembl_havana gene 146116002 148420998 . + .
## 2 9 ensembl_havana gene 8314246 10612723 . - .
## 3 X ensembl_havana gene 31097677 33339441 . - .
## 4 11 ensembl_havana gene 83455012 85627922 . - .
## 5 8 ensembl_havana gene 2935353 4994972 . - .
## 6 20 ensembl_havana gene 13995369 16053197 . + .
## gene_name gene_id
## 1 CNTNAP2 ENSG00000174469
## 2 PTPRD ENSG00000153707
## 3 DMD ENSG00000198947
## 4 DLG2 ENSG00000150672
## 5 CSMD1 ENSG00000183117
## 6 MACROD2 ENSG00000172264
## desc
## 1 contactin associated protein-like 2 [Source:HGNC Symbol%3BAcc:HGNC:13830]
## 2 protein tyrosine phosphatase%2C receptor type D [Source:HGNC Symbol%3BAcc:HGNC:9668]
## 3 dystrophin [Source:HGNC Symbol%3BAcc:HGNC:2928]
## 4 discs large MAGUK scaffold protein 2 [Source:HGNC Symbol%3BAcc:HGNC:2901]
## 5 CUB and Sushi multiple domains 1 [Source:HGNC Symbol%3BAcc:HGNC:14026]
## 6 MACRO domain containing 2 [Source:HGNC Symbol%3BAcc:HGNC:16126]
## length
## 1 2304997
## 2 2298478
## 3 2241765
## 4 2172911
## 5 2059620
## 6 2057829
head(arrange(ndf, length))
## seqid source type start end score strand phase gene_name
## 1 14 havana gene 22438547 22438554 . + . TRDD1
## 2 14 havana gene 22439007 22439015 . + . TRDD2
## 3 7 havana gene 142786213 142786224 . + . TRBD1
## 4 14 havana gene 22449113 22449125 . + . TRDD3
## 5 4 havana gene 10238213 10238235 . - . AC006499.9
## 6 3 havana gene 179452395 179452419 . - . RP11-145M9.2
## gene_id
## 1 ENSG00000223997
## 2 ENSG00000237235
## 3 ENSG00000282431
## 4 ENSG00000228985
## 5 ENSG00000271544
## 6 ENSG00000239255
## desc
## 1 T cell receptor delta diversity 1 [Source:HGNC Symbol%3BAcc:HGNC:12254]
## 2 T cell receptor delta diversity 2 [Source:HGNC Symbol%3BAcc:HGNC:12255]
## 3 T cell receptor beta diversity 1 [Source:HGNC Symbol%3BAcc:HGNC:12158]
## 4 T cell receptor delta diversity 3 [Source:HGNC Symbol%3BAcc:HGNC:12256]
## 5
## 6
## length
## 1 8
## 2 9
## 3 12
## 4 13
## 5 23
## 6 25
Gene Distribution Among Chromosomes
The number of genes per chromosome are counted with the “subset()”, “table()” and “sort()” functions as described earlier.
Python:
ndf = ndf[ndf.seqid.isin(chrs)]
chr_gene_counts = ndf.groupby('seqid').count().ix[:, 0].sort_values().ix[::-1]
chr_gene_counts
R:
ndf$seqid <- as.character(ndf$seqid) # as factors it will subset the dataframe but keep the factor levels
ndf <- subset(ndf, seqid %in% chrs)
chr_gene_counts <- sort(table(ndf$seqid), decreasing = TRUE)
chr_gene_counts
##
## 1 2 11 19 17 3 6 12 7 5 16 X 4 9 8
## 3902 2806 2561 2412 2280 2204 2154 2140 2106 2002 1881 1852 1751 1659 1628
## 10 15 14 22 20 13 18 21 Y
## 1600 1476 1449 996 965 872 766 541 436
To see all genes that are on mitochondrial chromosome, we subset the first dataframe by two conditions. In both, R and Python this is done with the ampersand symbol but in R we don’t need brackets around the individual conditions.
Python:
df[(df.type == 'gene') & (df.seqid == 'MT')]
R:
subset(df, type == "gene" & seqid == "MT")
## seqid source type start end score strand phase
## 2514004 MT insdc gene 648 1601 . + .
## 2514010 MT insdc gene 1671 3229 . + .
## 2514017 MT insdc gene 3307 4262 . + .
## 2514030 MT insdc gene 4470 5511 . + .
## 2514049 MT insdc gene 5904 7445 . + .
## 2514059 MT insdc gene 7586 8269 . + .
## 2514066 MT insdc gene 8366 8572 . + .
## 2514070 MT insdc gene 8527 9207 . + .
## 2514074 MT insdc gene 9207 9990 . + .
## 2514081 MT insdc gene 10059 10404 . + .
## 2514088 MT insdc gene 10470 10766 . + .
## 2514092 MT insdc gene 10760 12137 . + .
## 2514105 MT insdc gene 12337 14148 . + .
## 2514109 MT insdc gene 14149 14673 . - .
## 2514116 MT insdc gene 14747 15887 . + .
## attributes
## 2514004 ID=gene:ENSG00000211459;Name=MT-RNR1;biotype=Mt_rRNA;description=mitochondrially encoded 12S RNA [Source:HGNC Symbol%3BAcc:HGNC:7470];gene_id=ENSG00000211459;logic_name=mt_genbank_import;version=2
## 2514010 ID=gene:ENSG00000210082;Name=MT-RNR2;biotype=Mt_rRNA;description=mitochondrially encoded 16S RNA [Source:HGNC Symbol%3BAcc:HGNC:7471];gene_id=ENSG00000210082;logic_name=mt_genbank_import;version=2
## 2514017 ID=gene:ENSG00000198888;Name=MT-ND1;biotype=protein_coding;description=mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 1 [Source:HGNC Symbol%3BAcc:HGNC:7455];gene_id=ENSG00000198888;logic_name=mt_genbank_import;version=2
## 2514030 ID=gene:ENSG00000198763;Name=MT-ND2;biotype=protein_coding;description=mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 2 [Source:HGNC Symbol%3BAcc:HGNC:7456];gene_id=ENSG00000198763;logic_name=mt_genbank_import;version=3
## 2514049 ID=gene:ENSG00000198804;Name=MT-CO1;biotype=protein_coding;description=mitochondrially encoded cytochrome c oxidase I [Source:HGNC Symbol%3BAcc:HGNC:7419];gene_id=ENSG00000198804;logic_name=mt_genbank_import;version=2
## 2514059 ID=gene:ENSG00000198712;Name=MT-CO2;biotype=protein_coding;description=mitochondrially encoded cytochrome c oxidase II [Source:HGNC Symbol%3BAcc:HGNC:7421];gene_id=ENSG00000198712;logic_name=mt_genbank_import;version=1
## 2514066 ID=gene:ENSG00000228253;Name=MT-ATP8;biotype=protein_coding;description=mitochondrially encoded ATP synthase 8 [Source:HGNC Symbol%3BAcc:HGNC:7415];gene_id=ENSG00000228253;logic_name=mt_genbank_import;version=1
## 2514070 ID=gene:ENSG00000198899;Name=MT-ATP6;biotype=protein_coding;description=mitochondrially encoded ATP synthase 6 [Source:HGNC Symbol%3BAcc:HGNC:7414];gene_id=ENSG00000198899;logic_name=mt_genbank_import;version=2
## 2514074 ID=gene:ENSG00000198938;Name=MT-CO3;biotype=protein_coding;description=mitochondrially encoded cytochrome c oxidase III [Source:HGNC Symbol%3BAcc:HGNC:7422];gene_id=ENSG00000198938;logic_name=mt_genbank_import;version=2
## 2514081 ID=gene:ENSG00000198840;Name=MT-ND3;biotype=protein_coding;description=mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 3 [Source:HGNC Symbol%3BAcc:HGNC:7458];gene_id=ENSG00000198840;logic_name=mt_genbank_import;version=2
## 2514088 ID=gene:ENSG00000212907;Name=MT-ND4L;biotype=protein_coding;description=mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 4L [Source:HGNC Symbol%3BAcc:HGNC:7460];gene_id=ENSG00000212907;logic_name=mt_genbank_import;version=2
## 2514092 ID=gene:ENSG00000198886;Name=MT-ND4;biotype=protein_coding;description=mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 4 [Source:HGNC Symbol%3BAcc:HGNC:7459];gene_id=ENSG00000198886;logic_name=mt_genbank_import;version=2
## 2514105 ID=gene:ENSG00000198786;Name=MT-ND5;biotype=protein_coding;description=mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 5 [Source:HGNC Symbol%3BAcc:HGNC:7461];gene_id=ENSG00000198786;logic_name=mt_genbank_import;version=2
## 2514109 ID=gene:ENSG00000198695;Name=MT-ND6;biotype=protein_coding;description=mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 6 [Source:HGNC Symbol%3BAcc:HGNC:7462];gene_id=ENSG00000198695;logic_name=mt_genbank_import;version=2
## 2514116 ID=gene:ENSG00000198727;Name=MT-CYB;biotype=protein_coding;description=mitochondrially encoded cytochrome b [Source:HGNC Symbol%3BAcc:HGNC:7427];gene_id=ENSG00000198727;logic_name=mt_genbank_import;version=2
We can get the chromosome lengths from the dataframe as well. We again subset to only the main chromosomes, then drop unwanted columns and order by length.
Python:
gdf = gdf[gdf.seqid.isin(chrs)]
gdf.drop(['start', 'end', 'score', 'strand', 'phase' ,'attributes'], axis=1, inplace=True)
gdf.sort_values('length').ix[::-1]
R:
gdf$seqid <- as.character(gdf$seqid) # as factors it will subset the dataframe but keep the factor levels
gdf <- subset(gdf, as.character(seqid) %in% chrs) %>%
select(-(start:attributes))
arrange(gdf, desc(length))
## seqid source type length
## 1 1 GRCh38 chromosome 248956422
## 2 2 GRCh38 chromosome 242193529
## 3 3 GRCh38 chromosome 198295559
## 4 4 GRCh38 chromosome 190214555
## 5 5 GRCh38 chromosome 181538259
## 6 6 GRCh38 chromosome 170805979
## 7 7 GRCh38 chromosome 159345973
## 8 X GRCh38 chromosome 156040895
## 9 8 GRCh38 chromosome 145138636
## 10 9 GRCh38 chromosome 138394717
## 11 11 GRCh38 chromosome 135086622
## 12 10 GRCh38 chromosome 133797422
## 13 12 GRCh38 chromosome 133275309
## 14 13 GRCh38 chromosome 114364328
## 15 14 GRCh38 chromosome 107043718
## 16 15 GRCh38 chromosome 101991189
## 17 16 GRCh38 chromosome 90338345
## 18 17 GRCh38 chromosome 83257441
## 19 18 GRCh38 chromosome 80373285
## 20 20 GRCh38 chromosome 64444167
## 21 19 GRCh38 chromosome 58617616
## 22 Y GRCh38 chromosome 54106423
## 23 22 GRCh38 chromosome 50818468
## 24 21 GRCh38 chromosome 46709983
## 25 MT GRCh38 chromosome 16569
Now, we merge the dataframe with the number of genes per chromosome with the dataframe of chromosome lengths. Because R’s “table()” function produces a vector, we need to convert it to a dataframe first and define the column names. Then, we use the “merge()” function and point to the name of the column we want to merge by.
Python:
cdf = chr_gene_counts.to_frame(name='gene_count').reset_index()
cdf.head(2)
merged = gdf.merge(cdf, on='seqid')
R:
cdf <- as.data.frame(chr_gene_counts)
colnames(cdf) <- c("seqid", "gene_count")
head(cdf, n = 2)
## seqid gene_count
## 1 1 3902
## 2 2 2806
merged <- merge(gdf, cdf, by = "seqid")
merged
## seqid source type length gene_count
## 1 1 GRCh38 chromosome 248956422 3902
## 2 10 GRCh38 chromosome 133797422 1600
## 3 11 GRCh38 chromosome 135086622 2561
## 4 12 GRCh38 chromosome 133275309 2140
## 5 13 GRCh38 chromosome 114364328 872
## 6 14 GRCh38 chromosome 107043718 1449
## 7 15 GRCh38 chromosome 101991189 1476
## 8 16 GRCh38 chromosome 90338345 1881
## 9 17 GRCh38 chromosome 83257441 2280
## 10 18 GRCh38 chromosome 80373285 766
## 11 19 GRCh38 chromosome 58617616 2412
## 12 2 GRCh38 chromosome 242193529 2806
## 13 20 GRCh38 chromosome 64444167 965
## 14 21 GRCh38 chromosome 46709983 541
## 15 22 GRCh38 chromosome 50818468 996
## 16 3 GRCh38 chromosome 198295559 2204
## 17 4 GRCh38 chromosome 190214555 1751
## 18 5 GRCh38 chromosome 181538259 2002
## 19 6 GRCh38 chromosome 170805979 2154
## 20 7 GRCh38 chromosome 159345973 2106
## 21 8 GRCh38 chromosome 145138636 1628
## 22 9 GRCh38 chromosome 138394717 1659
## 23 X GRCh38 chromosome 156040895 1852
## 24 Y GRCh38 chromosome 54106423 436
To calculate the correlation between length and gene count, we subset the merged dataframe to those two columns and use the “corr()” (Python) or “cor()” (R) function.
Python:
merged[['length', 'gene_count']].corr()
R:
cor(merged[, c("length", "gene_count")])
## length gene_count
## length 1.0000000 0.7282208
## gene_count 0.7282208 1.0000000
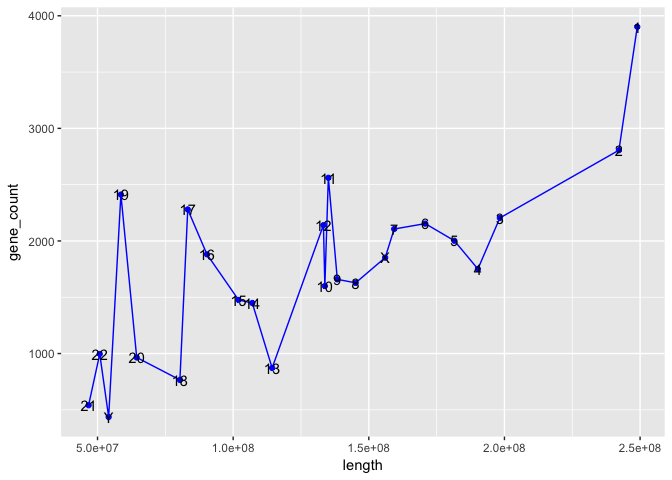
And now we produce the final plot: a line plot of chromosome length by number of genes per chromosome. For Python, we again use the matplotlib and for R the ggplot2 packages. Because Zhuyi Xue creates a new dataframe and tweaks the plot somewhat, our ggplot2 code is simpler and tidier here.
Python:
ax = merged[['length', 'gene_count']].sort_values('length').plot(x='length', y='gene_count', style='o-')
# add some margin to both ends of x axis
xlim = ax.get_xlim()
margin = xlim[0] * 0.1
ax.set_xlim([xlim[0] - margin, xlim[1] + margin])
# Label each point on the graph
for (s, x, y) in merged[['seqid', 'length', 'gene_count']].sort_values('length').values:
ax.text(x, y - 100, str(s))
R:
merged[, c("seqid", "length", "gene_count")] %>%
arrange(desc(length)) %>%
ggplot(aes(x = length, y = gene_count, label = seqid)) +
geom_point(color = "blue") +
geom_line(color = "blue") +
geom_text()
## R version 3.3.2 (2016-10-31)
## Platform: x86_64-apple-darwin13.4.0 (64-bit)
## Running under: macOS Sierra 10.12.1
##
## locale:
## [1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
##
## attached base packages:
## [1] stats graphics grDevices utils datasets methods base
##
## other attached packages:
## [1] ggplot2_2.2.1 dplyr_0.5.0
##
## loaded via a namespace (and not attached):
## [1] Rcpp_0.12.8 codetools_0.2-15 digest_0.6.11 rprojroot_1.1
## [5] assertthat_0.1 plyr_1.8.4 grid_3.3.2 R6_2.2.0
## [9] gtable_0.2.0 DBI_0.5-1 backports_1.0.4 magrittr_1.5
## [13] scales_0.4.1 evaluate_0.10 stringi_1.1.2 lazyeval_0.2.0
## [17] rmarkdown_1.3 labeling_0.3 tools_3.3.2 stringr_1.1.0
## [21] munsell_0.4.3 yaml_2.1.14 colorspace_1.3-2 htmltools_0.3.5
## [25] knitr_1.15.1 tibble_1.2



